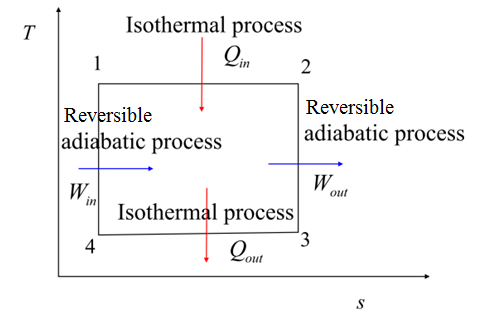
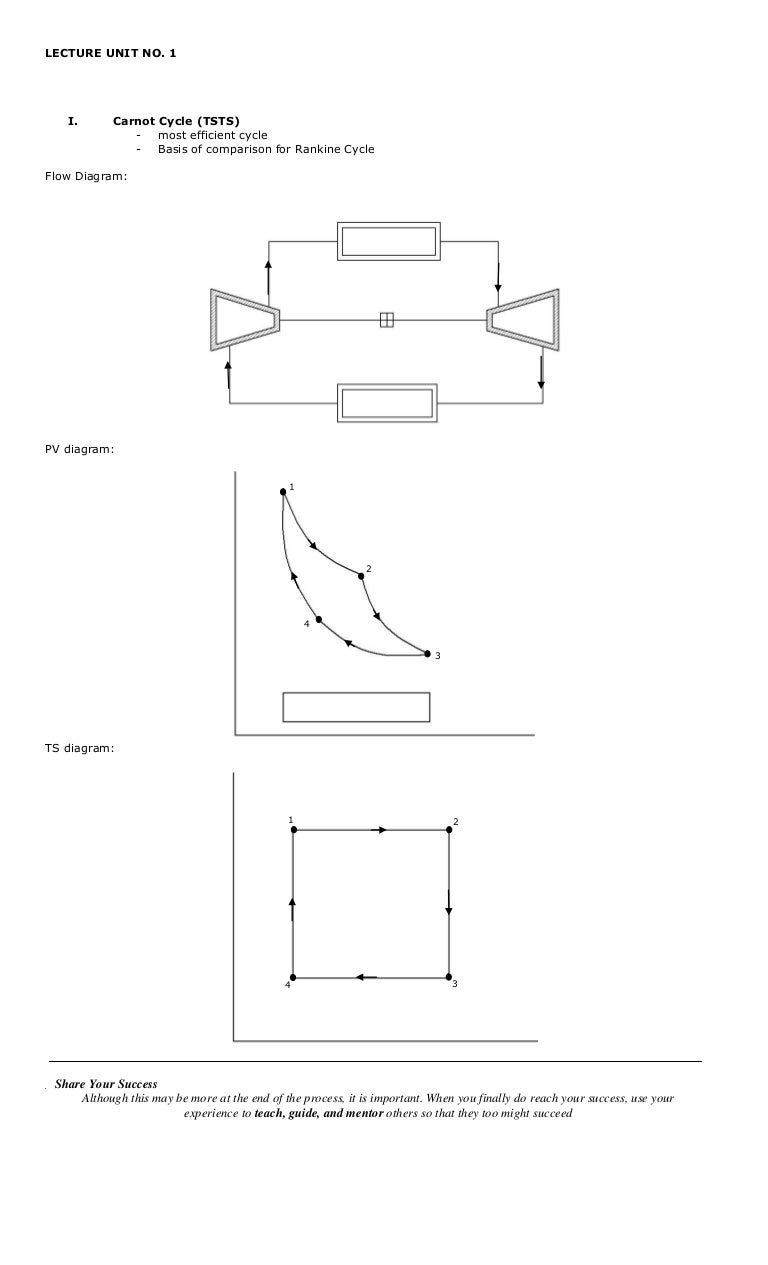
Consider A Carnot Cycle Executed In A Closed System
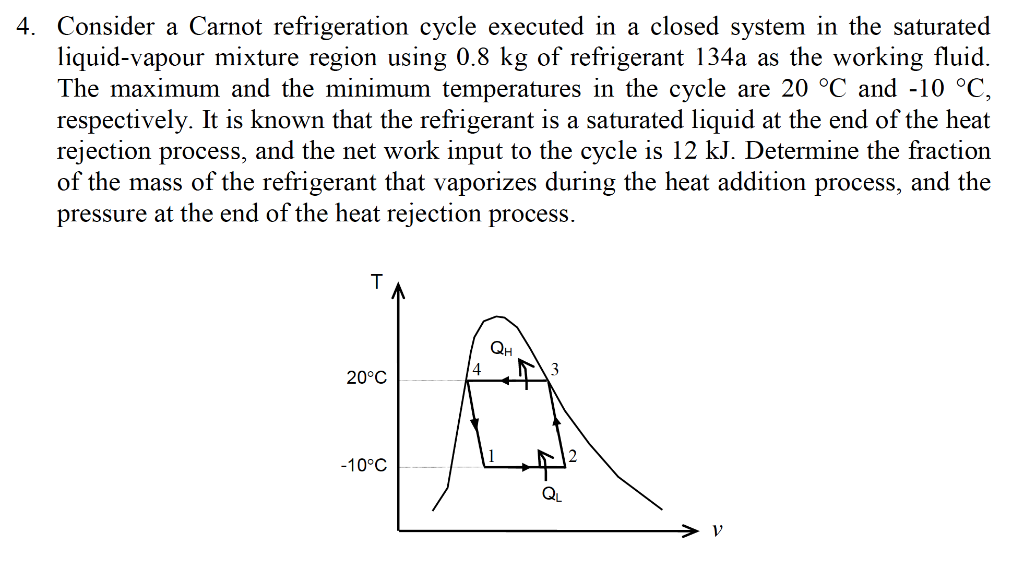
Consider a carnot cycle executed in a closed system. A Carnot refrigeration cycle executed in a closed system in the saturated liquid-vapor mixture region using 085 kg of refrigerant-134a as the working fluid. Consider a Carnot cycle executed in a closed system with 06 kg of air. The maximum pressure in the cycle is 1300 kPa while the maximum temperature is 950 K.
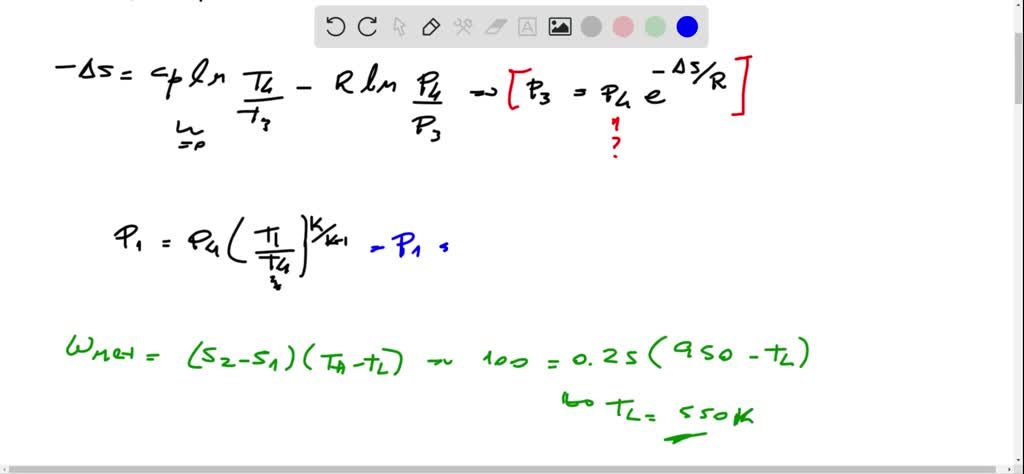
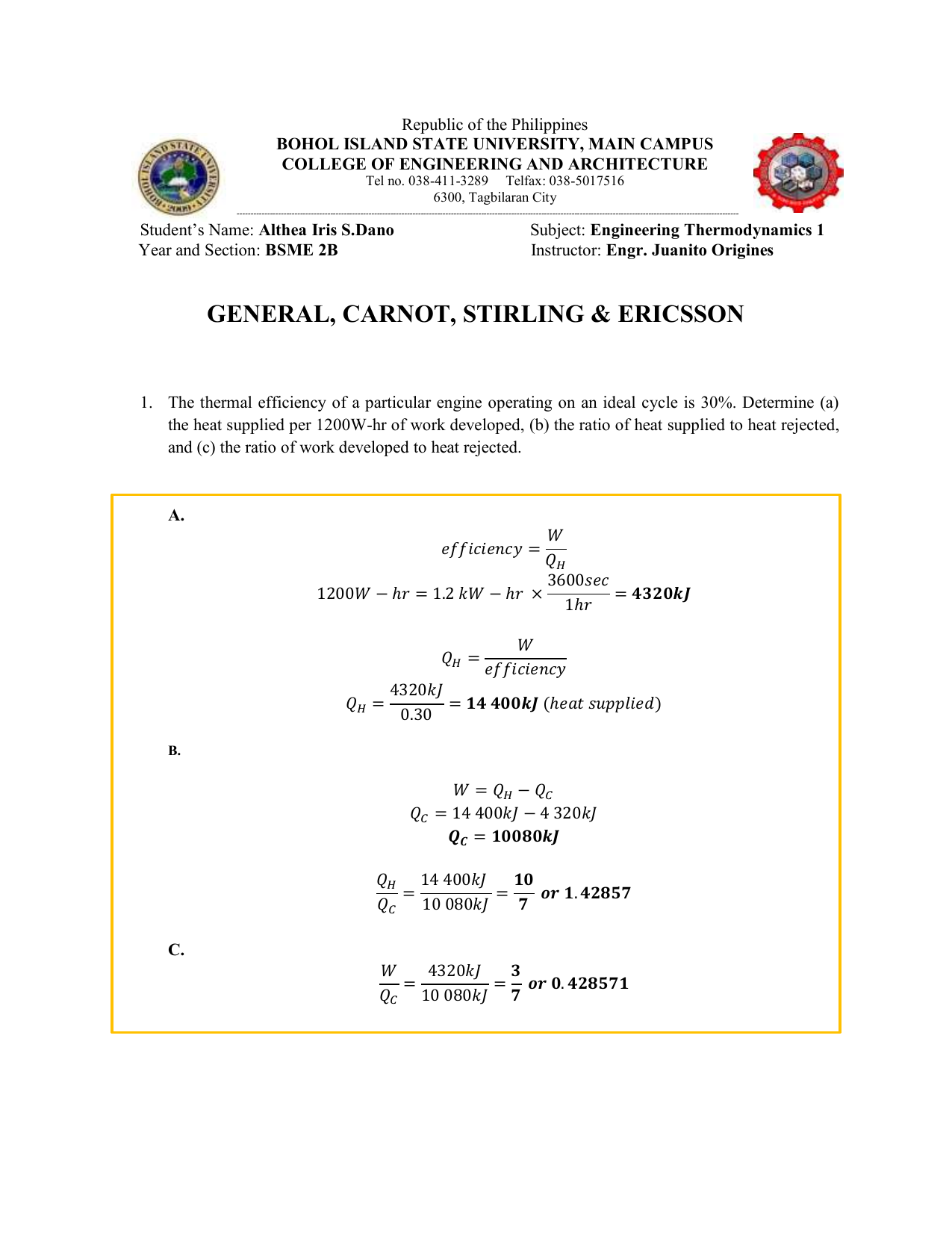
The pressures before and after the isothermal compression are 150 and 300 kPa respectively. Consider a Carnot cycle executed in a closed system with air as the working fluid. It is known that the maximum absolute temperature in the cycle is twice the minimum absolute temperature and the net work output of the cycle is 25 kJ.
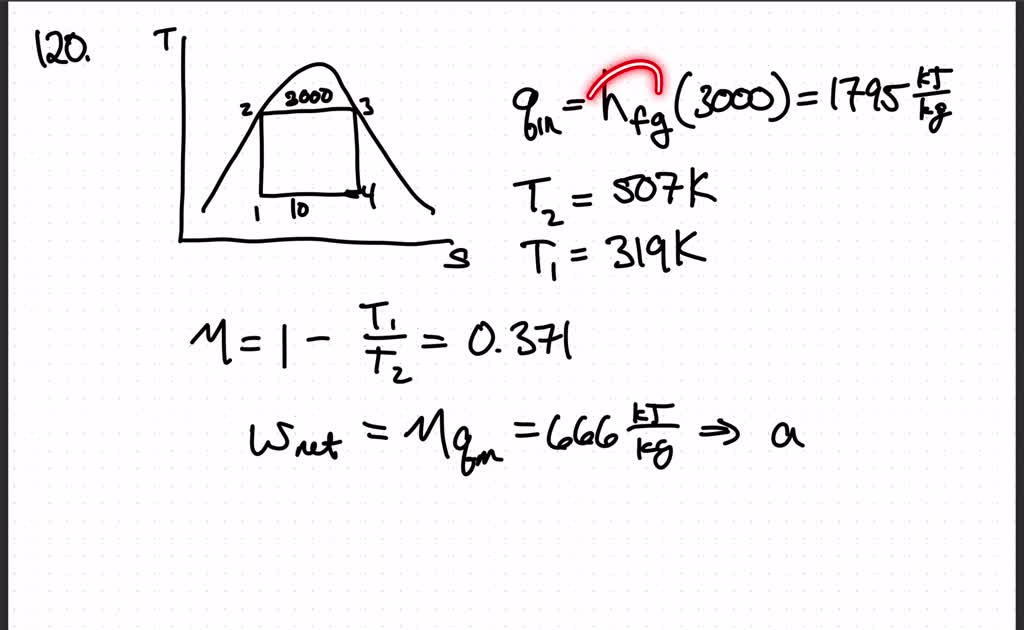
Consider a Carnot heat-engine cycle executed in a closed system using 00103 kg of steam as the working fluid. Consider a Carnot refrigeration cycle executed in a closed system in the saturated liquidvapor mixture region using 106 kg of refrigerant-134a as the working fluid. Assuming constant specific heats determine the net work output per cycle.
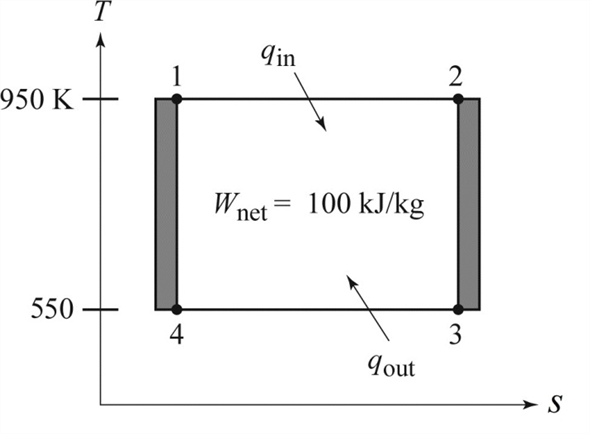
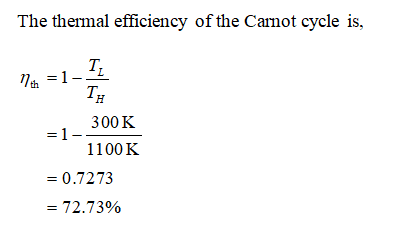
The temperature limits of the cycle are 300 and 900 K and the minimum and maximum pressures that occur during the cycle are 20 and 2000 kPa. Carnot cycle with the specified temperature limits is considered. The maximum pressure in the cycle is 1300 kPa while the maximum temperature is 950 K.
Consider a Carnot cycle executed in a closed system with 08 kg of air. Consider a Carnot refrigeration cycle executed in a closed system in the saturated liquidvapor mixture region using 096kg of refrigerant-134a as the working fluid. If the entropy increase during the isothermal heat rejection process is.
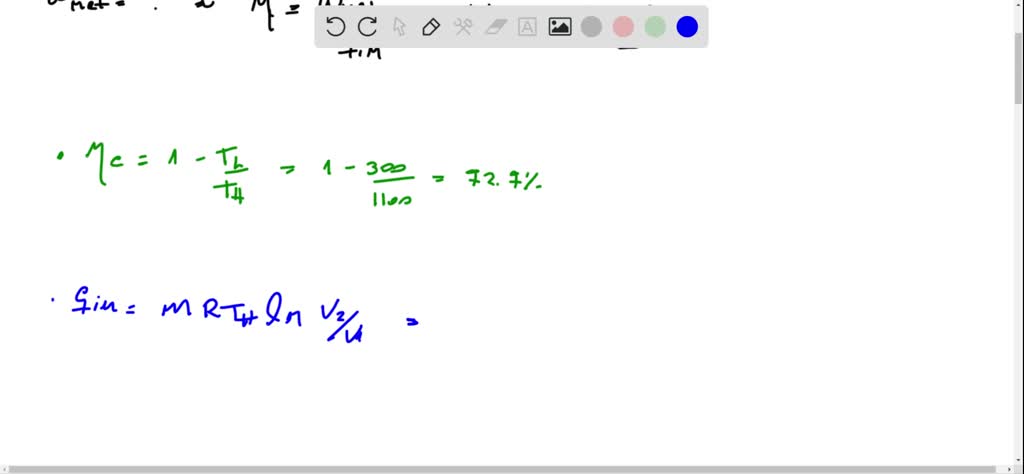
It is known that the maximum absolute temperature in the cycle is 12 times the minimum absolute temperature and the net work input to the cycle is 22 kJ. 1 Answer to Consider a Carnot cycle executed in a closed system with air as the working fluid. An air standard Carnot cycle is executed in a closed system between the temperature limits of 350 K and 1200 K.
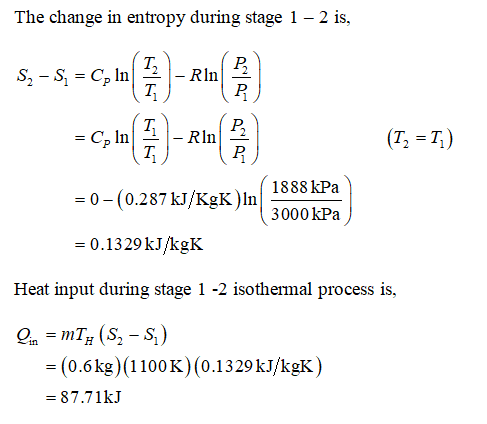
7-2-4 tmax-1200K An air standard Carnot cycle is executed in a closed system between the temperature limits of 350 K and 1200 K. The pressure before and after the isothermal compression are 150 kPa and 300 kPa respectively.
Assuming constant specific heats determine the net work output per cycle.
An air standard Carnot cycle is executed in a closed system between the temperature limits of 350 K and 1200 K. Consider a Carnot heat-engine cycle executed in a closed system using 0025 kg of steam as the working fluid. If the net work output per cycle is 05 kJ determine a the maximum pressure in the cycle. It is known that the maximum absolute temperature in the cycle is twice the minimum absolute temperature and the net work output of the cycle is 60 kJ. The maximum pressure in the cycle is 1300 kPa while the maximum temperature is 950 K. It is known that the maximum absolute temperature in the cycle is 12 times the minimum absolute temperature and the net work input to the cycle is 22 kJ. The temperature limits of the cycle are 300 and 1100 K and the minimum and maximum pressures that. Consider a Carnot heat-pump cycle executed in a steady-flow system in the saturated liquid-vapor mixture region using refrigerant-134a flowing at a rate of 022 mathrmkg. It is known that the maximum absolute temperature in the cycle is twice the minimum absolute temperature and the net work output of the cycle is 25 kJ.
State the assumptions clearly. The temperature limits of the-cycle are 300 and 1100 K and the minimum and maximum pressures that occur during the cycle are 20. Consider a Carnot cycle executed in a closed system with 06 kg of air. The temperature limits of the cycle are 300 and 1100 K and the minimum and maximum pressures that occur during the cycle are 20 and 3000 kPa. The maximum pressure in the cycle is 1300 kPa while the maximum temperature is 950 K. State the assumptions clearly. Assuming constant specific heats determine the net work output per cycle.
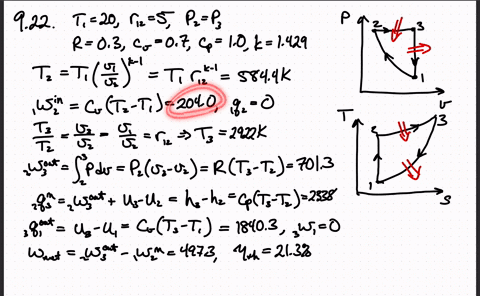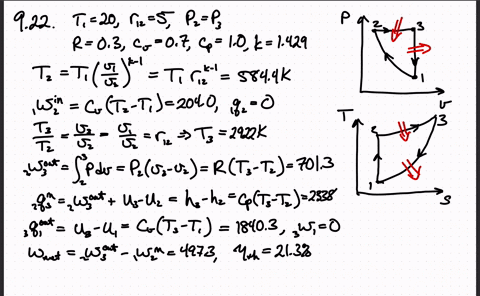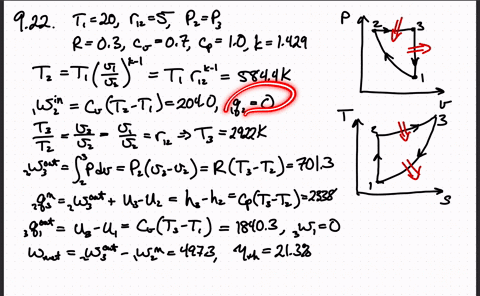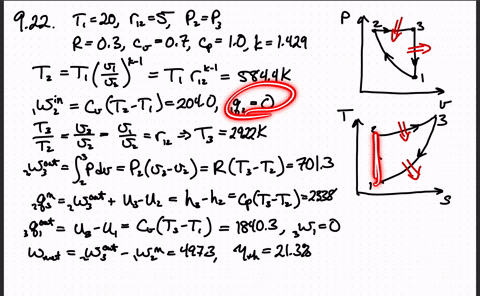


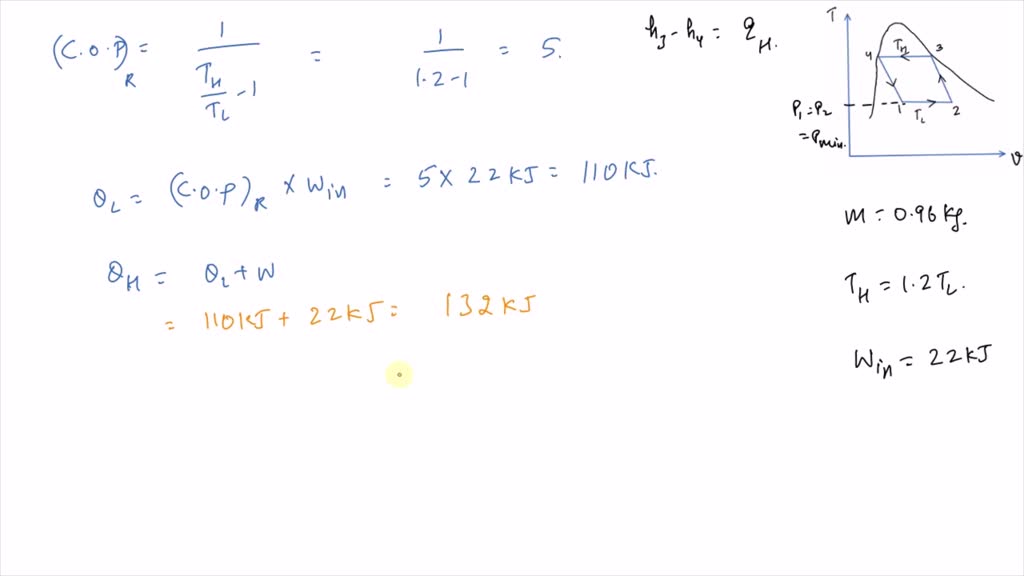
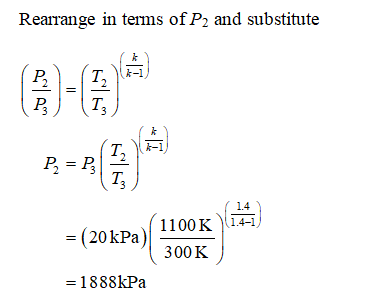
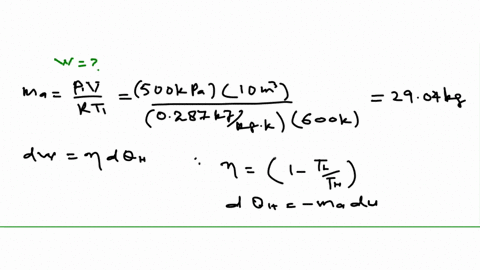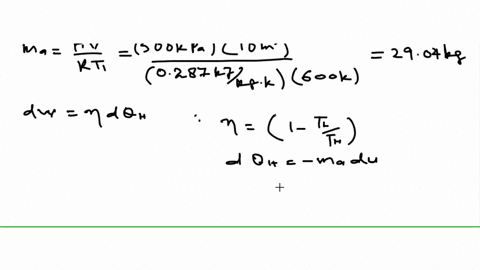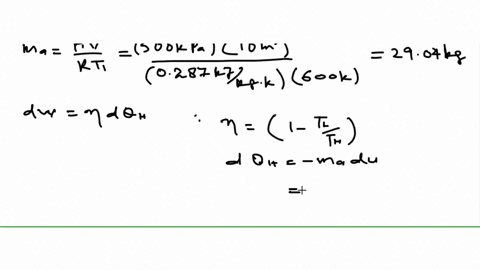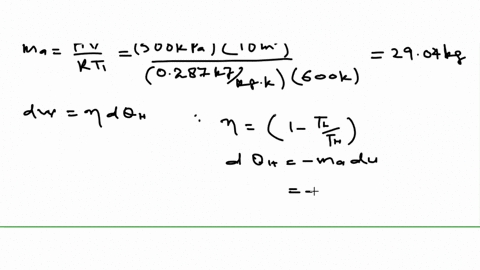























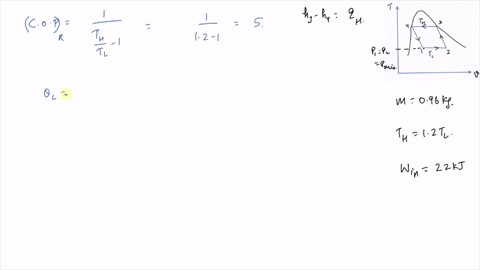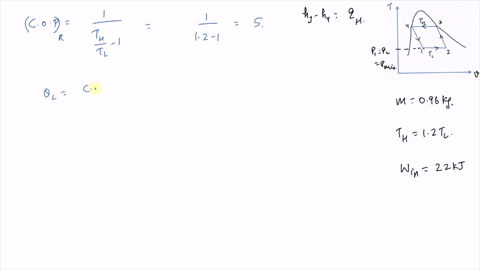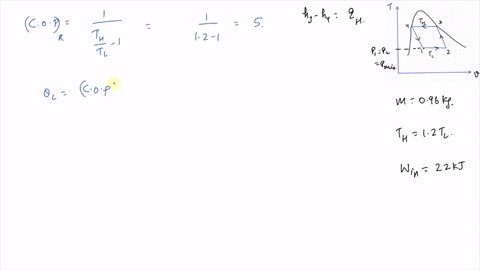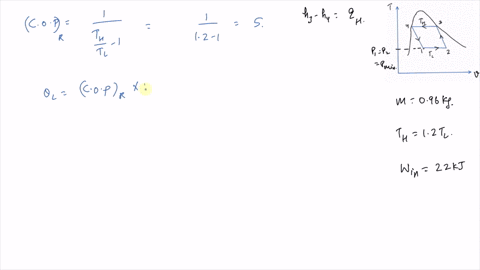
Post a Comment for "Consider A Carnot Cycle Executed In A Closed System"